Health care information
Visit your patient portal
Make payments, submit copays, see your billing statements, view test results, schedule appointments and message your care team.
Health finances
Make your health care dollars go further
You’ll be surprised at all the everyday health items you can buy with your HSA and FSA card — saving up to 30% with pretax dollars.


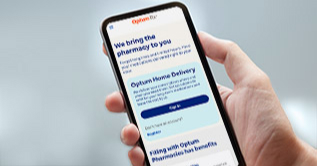
Online pharmacy
You’ve got better things to do than stand in line at the pharmacy
Optum Home Delivery Pharmacy brings your medications directly to your door and gives you 24/7 access to our licensed pharmacists — ready to answer any question.
A healthier you happens here
Find tips, ideas and information to inspire healthy living at any age, including topics important to you and your family.

Healthy living
Your age-by-age guide to annual checkups
It’s a good idea to see your doctor regularly at any age. But what happens during those visits changes as you get older. Here’s what to know.

Healthy living
7 reasons to make time for your flu vaccine
Getting an annual flu vaccine can help protect you, your loved ones and others. And it’s quick and convenient, too.

Your care
5 surprising ways your Medicare plan helps you stay healthy
Learn how your health plan and your Optum care team make a winning combination.
Video
See how we’re creating the care you want so you can live better
As a leading health solution and care delivery organization, our work is complex, but our mission is simple. We’re creating a healthier world, with you at the center.
Join us
Explore Optum jobs
Check out current career opportunities to discover how you can start caring, connecting and growing — together with Optum.
Support
You’ve got questions, Optum has answers
Whether it’s about health care, financial or pharmacy-related services, we’ll help you find the answer you need.